‘Gold Rush’ – Natural Hydrogen
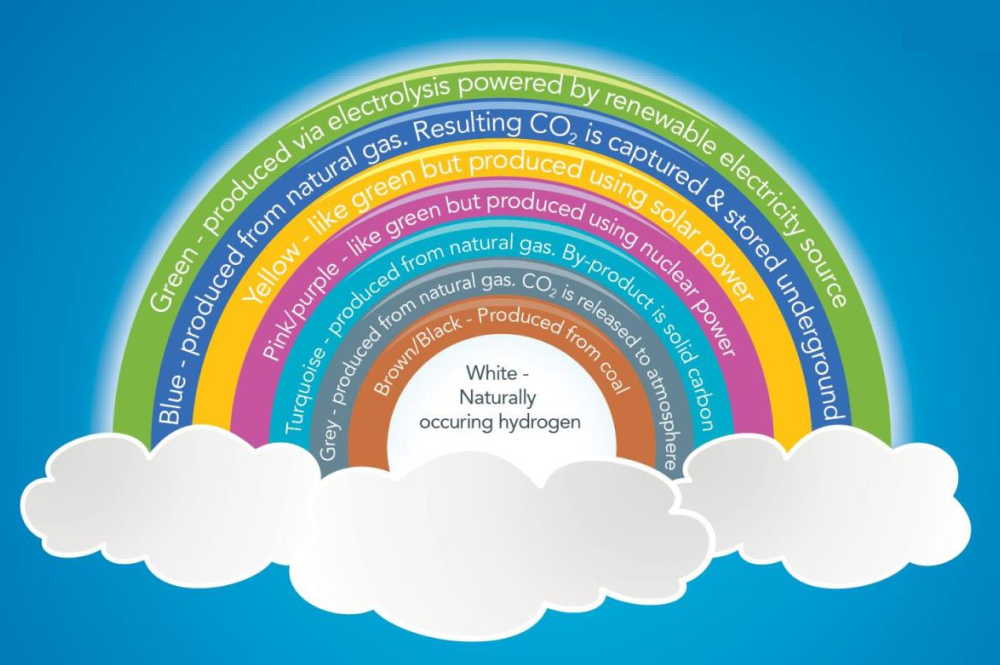
Hydrogen exists naturally in nature. You would most commonly see it in the molecule for water; H2O. The H2 represents two hydrogen atoms. It is the most abundant element on earth and can be found most commonly bound up with other substances such as water or methane. There are many ways to create hydrogen, also known as the hydrogen rainbow.
What we see most discussed is the production of hydrogen using renewable technologies, known as green hydrogen. The further down the rainbow you travel, the less valuable producing hydrogen becomes. Who would want to produce hydrogen by burning coal?
At the very bottom is naturally occurring hydrogen. This is usually linked to water, methane and propane. An unpublished study by the US Geological Survey states that as much as 5trn tonnes of hydrogen exists in underground reservoirs around the world. Though some would question such a statement from an unpublished document. In 2023, the US Department of Energy (DoE) announced $20m to explore the potential of geologic hydrogen.
Models by USGS research geologist Geoffrey Ellis predict that global hydrogen in this form could supply demand for thousands of years. However, he warns that most of this hydrogen is likely to be inaccessible to be economically viable for extraction. Ellis explains “to begin to understand the potential for hydrogen accumulation, scientists need a better geologic model to understand how the hydrogen forms, where it comes from within the rock layers, and where it ends up”.
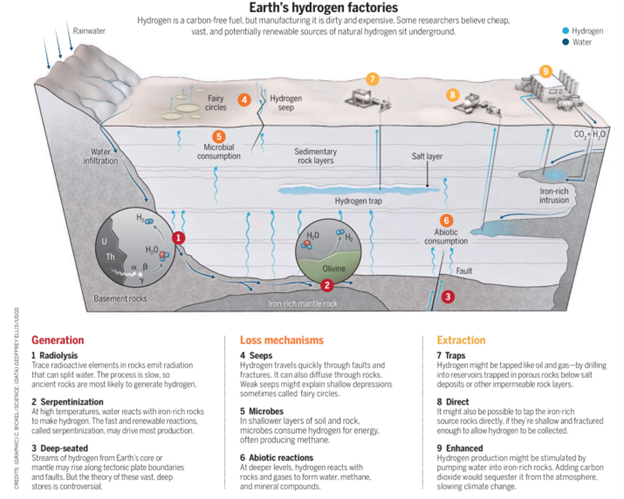
To achieve this, Ellis is using the petroleum system approach.
The petroleum system is a conceptual model designed for understanding the occurrence of petroleum within geologic basins. It has been used by petroleum geologists for decades to effectively guide oil and gas exploration and to derive accurate assessments of undiscovered petroleum resources.
This system is then adapted to the hydrogen system, where geologists have to understand three key areas:
- How natural hydrogen forms within rock layers
- What types of natural processes might affect the hydrogen once formed
- How hydrogen can get trapped in rock layers along its way to the surface
Hydrogen is generated through many natural processes. However, there is a need to understand which of these are capable of generating quantities large enough to make it viable. There are a number of natural processes that consume the gas once it is formed. Hydrogen that isn’t consumed may reach porous rock where it could form gas accumulation. But for this to occur, there must be what’s known as seal rocks in place. For those that know their periodic table, hydrogen is the lightest element making it hard to contain and store. On this basis, the assumption by geologists has been that seal rocks would not work and the hydrogen would escape. Contrary to that long standing knowledge, studies have shown “that the diameter of a molecule of two hydrogen atoms is about equal to that of a single helium atom and that the two gases are likely to get trapped by similar rock layers”. As helium can get trapped, there is an argument that hydrogen can as well. It is these facts that are being incorporated into the models to understand resource levels of naturally occurring hydrogen.
The idea of being able to extract hydrogen from the earth provides a lot of hope in meeting net zero plans of countries and organisations. However, it is still a long way off and requires further modelling and exploration.


